The adrenal steroid dehydroepiandrosterone (DHEA) may improve vascular function, but the mechanism is unclear. In the present study, we show that DHEA significantly increased cell viability, reduced caspase-3 activity, and protected both bovine and human vascular endothelial cells against serum deprivation-induced apoptosis.
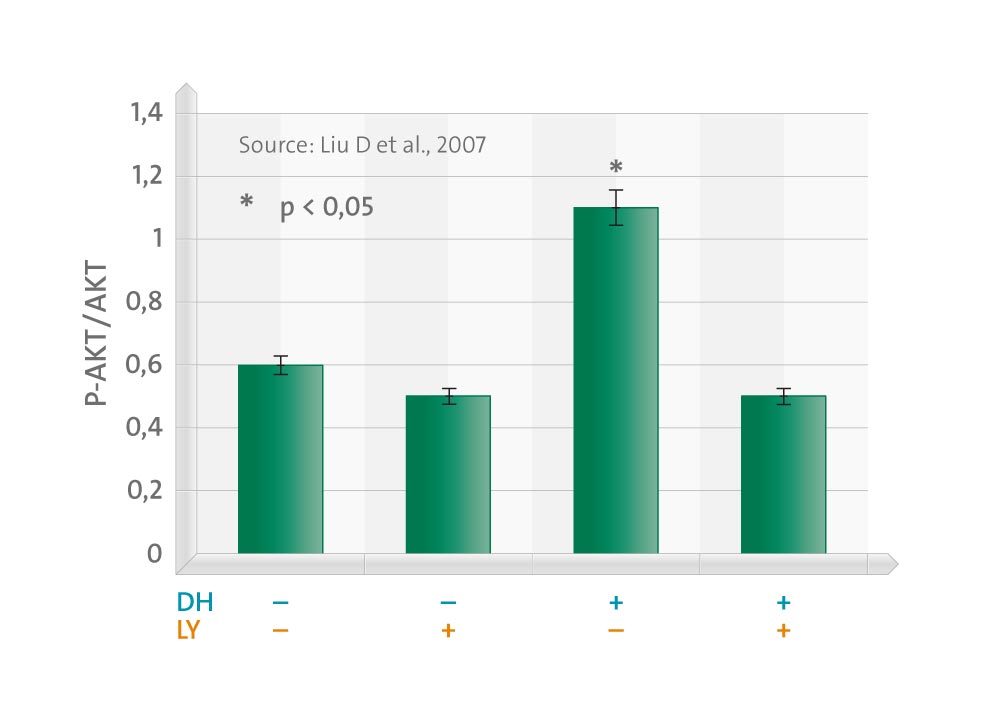
This effect was dose dependent and maximal at physiological concentrations (0.1-10 nM). DHEA stimulation of bovine aortic endothelial cells resulted in rapid and dose-dependent phosphorylation of Akt, which was blocked by LY294002, a specific inhibitor of phosphatidylinositol 3-kinase (PI3K), the upstream kinase of Akt. Accordingly, inhibition of PI3K or transfection of the cells with dominant-negative Akt ablated the antiapoptotic effect of DHEA. The induced Akt phosphorylation and subsequent cytoprotective effect of DHEA were dependent on activation of Galphai proteins, but were estrogen receptor independent, because these effects were blocked by pertussis toxin but not by the estrogen receptor inhibitor ICI182,780 or the aromatase inhibitor aminoglutethimide.
Finally, DHEA enhanced antiapoptotic Bcl-2 protein expression, its promoter activity, and gene transcription attributable to the activation of the PI3K/Akt pathway. Neutralization of Bcl-2 by antibody transfection significantly decreased the antiapoptotic effect of DHEA.
These findings provide the first evidence that DHEA acts as a survival factor for endothelial cells by triggering the Galphai-PI3K/Akt-Bcl-2 pathway to protect cells against apoptosis. This may represent an important mechanism underlying the vascular protective effect of DHEA.