Objective
This analysis was to investigate the effects of dehydroepiandrosterone (DHEA) on cardiovascular risk factors in older women with frailty characteristics.
Design, setting and participants
The study was a double-blind, randomised, placebo-controlled trial of 99 women (mean 76.6 +/- 6.0 year) with the low DHEA-S level and frailty.
Intervention
Participants received 50 mg/day DHEA or placebo for 6 months; all received calcium (1,000-1,200 mg/day diet) and supplement (combined) and cholecalciferol (1,000 IU/day). Women participated in 90-min twice weekly exercise regimens, either chair aerobics or yoga.
Main outcome measures
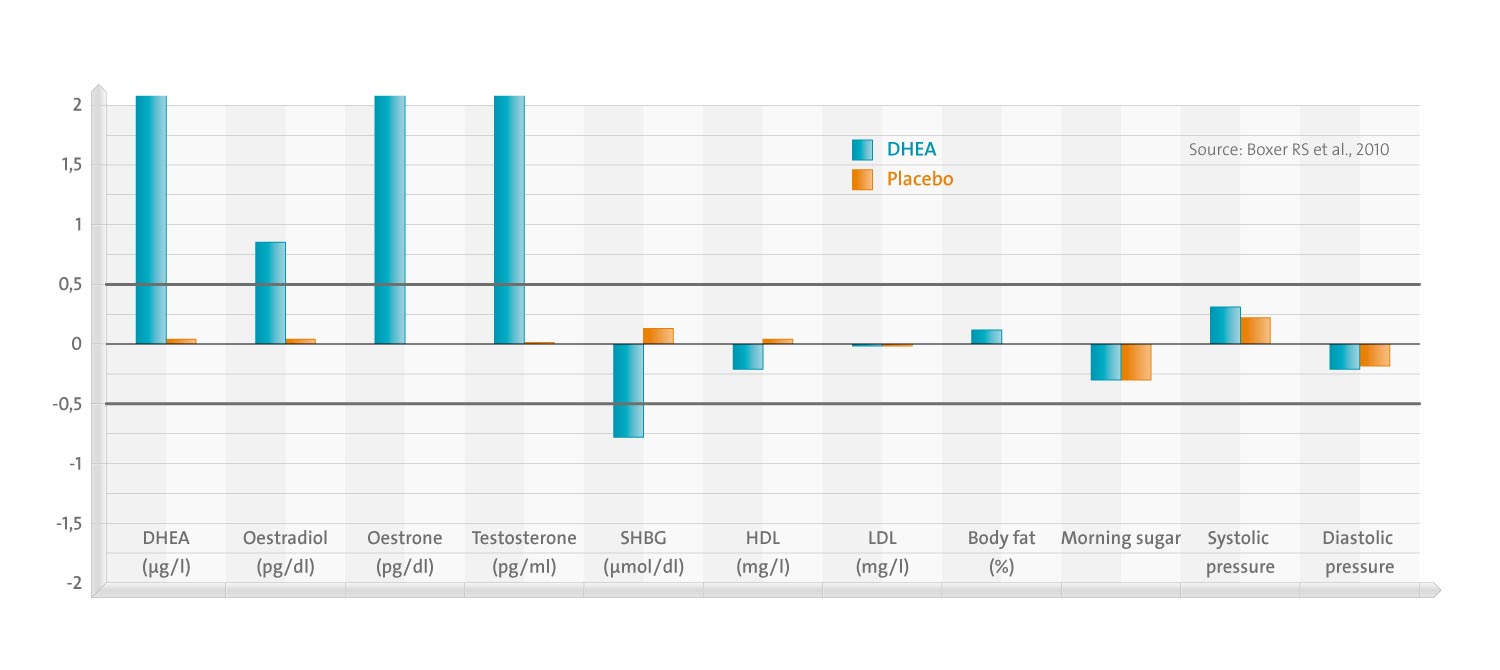
Assessment of outcome variables included hormone levels (DHEA-S, oestradiol, oestrone, testosterone and sex hormone-binding globulin (SHBG)), lipid profiles (total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and triglycerides), body composition measured by dual energy absorptiometry, glucose levels and blood pressure (BP).
Results
Eighty-seven women (88%) completed 6 months of study; 88% were pre-frail demonstrating 1-2 frailty characteristics and 12% were frail with > or =3 characteristics. There were significant changes in all hormone levels including DHEA-S, oestradiol, oestrone and testosterone and a decline in SHBG levels in those taking DHEA supplements. In spite of changes in hormone levels, there were no significant changes in cardiovascular risk factors including lipid profiles, body or abdominal fat, fasting glucose or BP.
Conclusion
Research to date has not shown consistent effects of DHEA on cardiovascular risk, and this study adds to the literature that short-term therapy with DHEA is safe for older women in relation to cardiovascular risk factors. This study is novel in that we recruited women with evidence of physical frailty.